Posted by Deena A. on 24th Feb 2024
What's the Deal with SLS?
We get lots of questions around SLS: is it good, is it bad? So I thought I would do a little research on this ingredient and provide a little snippet of the research that has been done regarding SLS. Scientific research is a lifelong journey, as new techniques and information come into play, things will change. Please do not consider this information as the final word, I just wanted to provide some of the information out there today.
What is it?
SLS known as Sodium Lauryl Sulphate
Soap and beauty products are often composed of a liquid made of a water phase and an oily phase. Oil and water don’t mix, so something is required to keep the ingredients together. This “something” is called a surfactant. A surfactant allows the oil and water molecules to bind together – it’s what’s found in soaps and detergents so we can wash our oily faces or dishes with water and get the grime to disappear. Sodium lauryl sulfate is a surfactant, and its efficacy, low cost, abundance and simplicity mean it’s used in a variety of cosmetic, dermatological and consumer products.
https://medicine.uq.edu.au/article/2019/12/what-s...
SLS has been an ingredient in shampoos since the 1930s. It works by trapping oil and dirt in hair so it can be rinsed away with water. SLS can help create a rich lather in products like body and hand wash, facial cleansers and bubble bath. SLS also helps create the foaming action in toothpaste and helps remove food particles from teeth.
https://www.chemicalsafetyfacts.org/chemicals/sod...
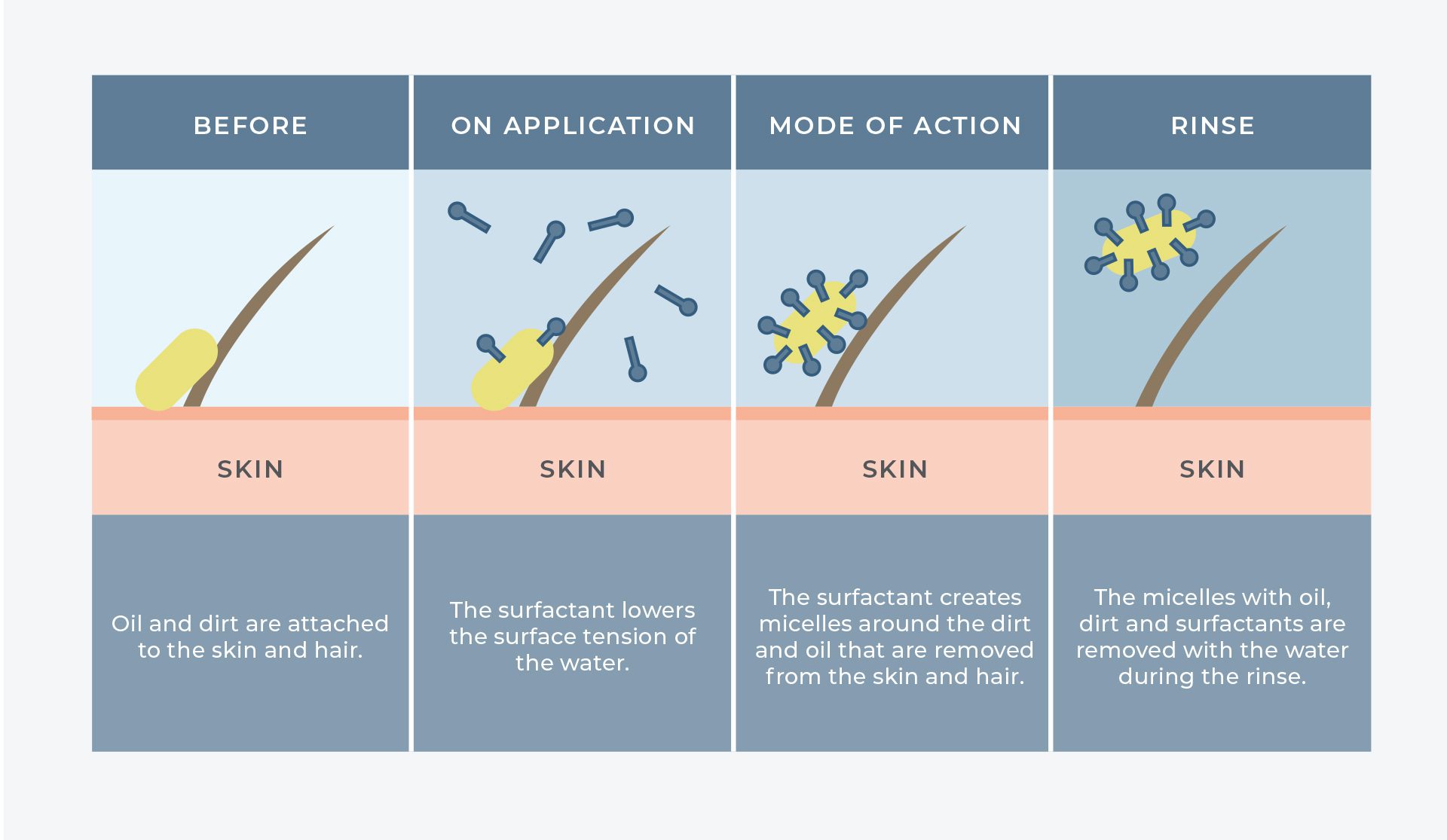
https://www.schoolofnaturalskincare.com/surfactant...
Are we concerned about it?
Since the early 1990s, misconstrued information on the human and environmental toxicity of SLS has led to consumer confusion and concern about the safety of SLS as an ingredient in household products. As scientific literature is inherently vulnerable to misinterpretation by the general public, health and safety claims made by marketing campaigns do not always align with the latest peer-reviewed scientific evidence. Oftentimes, consumer product claims use language in ways that can be misleading to the average consumer. Review of the human and environmental toxicity profiles of SLS is warranted to elucidate the known risks and benefits of using SLS in household cleaning product formulation.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4651...
It’s also important to note there’s no scientific evidence SLS causes cancer, despite what you may read on the internet. Multiple scientific bodies have reviewed SLS as an ingredient in personal care and cleaning products and determined its typical use in these applications to be safe for consumers and the environment.
https://www.chemicalsafetyfacts.org/chemicals/sod...
For SLS to be considered dangerous, it would have to be in contact with the skin for a long period of time. Generally, with consumer products such as washes that contain SLS, it’s assumed they won’t be on the skin for very long, meaning the chance of your skin being affected is pretty low. So authorities don’t ban its use, but instead cap the maximum percentage at which it can be used in products.
SLS can irritate the skin, eyes and respiratory tract and is toxic to aquatic organisms. While SLS is moderately toxic to aquatic life in its raw material form, product formulations that contain dilutions of SLS are not necessarily moderately toxic and, in fact, can be non toxic to aquatic life. However, the toxicity of SLS depends largely on the marine species, water hardness, and water temperature. By the time cleaning product ingredients reach natural waters, they are mostly degraded. Ecotoxicity studies have determined that a surfactant concentration of 0.5 mg/L of natural water would be essentially non toxic to fish and other aquatic life under most conditions. The ability of a chemical to decompose into simple, non toxic components under ambient environmental conditions within a short period of time (typically 96 hours) means that it’s biodegradable. SLS is readily biodegradable under aerobic and anaerobic conditions and, therefore, does not persist in the environment.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC46514...
Types of SLS
SLS can be synthetic or naturally derived. This chemical is synthesized by reacting lauryl alcohol from a petroleum or plant source with sulfur trioxide to produce hydrogen lauryl sulfate, which is then neutralized with sodium carbonate to produce SLS. From a sustainability and environmental health perspective, sourcing surfactants such as plant-derived SLS avoids incurring the additional environmental and human health impacts caused by the extraction of petroleum and the production of petrochemicals.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4651...
The use of SLS in cleaning product formulations does not introduce unnecessary risk to consumers or the environment because of the presence of the ingredient and if properly formulated and qualified, does not pose danger to human health and safety. Therefore, the perception that SLS is a threat to human health is not scientifically supported, and claims made to the contrary should be regarded as false and misleading.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4651...
Note, too, that every chemical has a toxic dose, and many common foods can be classified as toxic. For example, sodium chloride (table salt) has an LD50 of 3,000 mg/kg, making it moderately toxic by definition.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC46514...
Summary
It would seem that SLS derived from natural or synthetic sources is not a concern for humans, the environment or aquatic life with the regulations on the maximum amount per product enforced.
What are your thoughts on SLS? Are you concerned? When researching please make sure you look at good resources that are being reviewed by the scientific community and other environmental sources.

